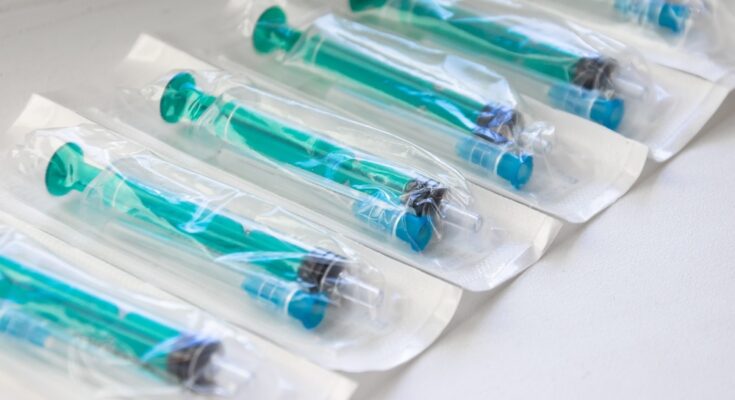
The final barrier protecting a sterile medical device from the outside world is its packaging. A tiny breach, invisible to the naked eye, can compromise a product’s sterility and put a patient at risk. That’s why rigorous package integrity validation is not just a regulatory hurdle—it’s a fundamental part of patient care. Continue reading to discover how packaging integrity validation is the key to patient safety.
Navigating Regulatory Standards
Regulatory bodies like the FDA and international organizations have specific standards for medical device packaging, such as ISO 11607. These regulations provide a framework for validating that a packaging system can maintain sterility from the point of manufacture to the moment of use. Complying with these standards is mandatory and demonstrates a commitment to quality and patient safety. The focus is on creating a documented process that proves the packaging consistently meets its specified requirements.
Identifying Common Package Defects
Package defects can come in many forms, including pinholes, channel leaks in seals, and tears. These issues often arise from manufacturing inconsistencies, problems with raw materials, or damage during shipping. Even a minuscule defect can create a pathway for microorganisms to enter, rendering the device non-sterile. The challenge often lies in detecting microleaks in medical device packaging, which are not always visible but pose a significant threat to the sterile barrier.
Essential Methods for Integrity Testing
To confirm a package’s integrity, medical device manufacturers use several recognized testing methods. Dye penetration testing, for example, involves applying a colored dye to the outside seal of a package to see if it seeps through any channels, making leaks visible. Burst testing is another common method where a package is inflated with air until it pops. This process helps determine the strength of the seals and identify the weakest point. These tests are critical for validating that the package is sealed correctly and can withstand pressure.
Best Practices for Package Integrity
Maintaining package integrity starts with a robust design and continues through the entire product lifecycle. It is vital to select the right materials that are compatible with both the device and the sterilization method. It’s also important to have a well-controlled manufacturing process to produce consistent, high-quality seals. Finally, routine testing and monitoring throughout production can help catch any deviations early, preventing defective packages from ever reaching the market.
The Future: AI in Package Inspection
Artificial intelligence is beginning to transform package integrity validation. AI-powered visual inspection systems can analyze packages with incredible speed and accuracy, spotting subtle defects that a human inspector might miss. This technology promises to make the process more efficient and reliable, further strengthening the sterile barrier.
By focusing on strong design, rigorous testing, and continuous monitoring, medical device manufacturers can uphold their most important responsibility: protecting patient health. For those looking to strengthen their validation processes, exploring advanced testing solutions can provide greater confidence in package integrity.